Describe the Electrical Charges of the Three Subatomic Particles
Protons neutrons and electrons. Where are each of the three particles located within the atom.
Nondestructive Evaluation Physics Atomic Elements
Electrons are the smallest of the three particles that make up atoms.

. Protons and neutrons have similar mass and all three particles combined equal the atomic mass. A subatomic particle that affects the mass of an atom and is found in the nucleus Atomic Mass The mass value in AMUs of the Protons plus the Neutrons in the nucleus of an atom. Neutrons have no electrical charge.
Describe the electrical charge mass and location of the three major subatomic particles in an atom. These sub atomic particles together constitute an atom and these atoms are the building blocks of elements or matter. Weve got the study and writing resources you need for your assignments.
An easy way to remember this is to remember that both proton and positive start with the letter P Neutrons have no electrical charge. Click to see full answer. The electron is a negatively charged particle present outside the nucleus whereas the proton is positively charged and is present inside the nucleus and finally the neutron is a neutrally charged particle present within.
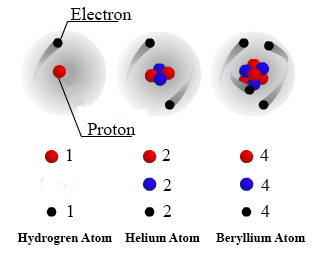
Protons neutrons and electronsprotons. It has a charge of 16 1019C. 3 Objective 1 Subatomic Particles Electron e -1 000055 0 Neutron n 0 100867 1 Proton p 1 100728 1 Mass Number Mass daltons Name Symbol Charge 4 Objective 2 Describe the basic structure of the atom and be able to define the following terms.
First week only 499. Protons neutrons and electrons are the three main sub-atomic particles which form an atom but only the later is an elementary particle as it is not divisible any further. Protons have a positive charge.
Electrons are negatively charged particles that are located outside the nucleus in orbit around the nucleus. Most of an atom is empty space. Subatomic particles and indicate the mass and electrical charge of each.
The nucleus of an atom contains not only protons but neutrons. Electrons are too small to matter when we look at mass. The charge of an electron is negative or The charge of proton is e or.
Solution for Describe the electrical charge mass and location of the threemajor subatomic particles in an atom. Which is largera proton or an atom of lead. 14 Votes Atoms are composed of three main subatomic particles.
Since the proton is a positively charged particle it has a positive charge. 3 subatomic particles. 455 770 Views.
Protons and neutrons are grouped together in the nucleus of an atom while electrons orbit about the. Which subatomic particle has a positive charge. These are three subatomic particles.
Protons neutrons and electrons are the three main subatomic particles found in an atom. Electrons revolves around an atom. Proton charge of e in the nucleus Neutron 0 charge in the nucleus and Electron charge of e outside the nucleus.
Electron charge negative one -1 neutron charge neutral 0 charge proton charge positive one 1. This is a positively charged particle that is present in the nucleus of atoms. There are far more than three known subatomic particlesHere are three of them and their electric charges Proton -- positive 1 Electron -- negative 1 Neutron --.
Protons neutrons and electrons. Neutrons as their name suggests is electrically neutral and hence has a neutral charge. Exercises Which is smalleran electron or a helium atom.
Subatomic particles and indicate the mass and electrical charge of each. What is the electrical charge of each particle. Electrons are electrically negative and therefore have a negative charge.
Most atoms have three different subatomic particles inside them. Electrons are found in shells or orbitals that surround the nucleus of an. Protons are positively charged species while neutrons are negatively charged species.
Protons neutrons and electrons. Negatively charged electrons orbit around a nucleus consisting of positively charged protons and uncharged neutrons. Positively charged mass 1673E located inside the nucleusneutrons.
Start your trial now. An atom consists of protons neutrons and electrons. But for ease we might say it has a charge of e or 1.
3 Objective 6 Electron e -1 000055 0 Neutron n 0 100867 1 Proton p 1 100728 1 Mass Number Mass Daltons Name Symbol Charge 4 Objective 7 Describe the basic structure of the atom and be able to define the following terms. The charges of all three subatomic particles are different. Protons neutrons and electrons are the three main subatomic particles found in an atom.
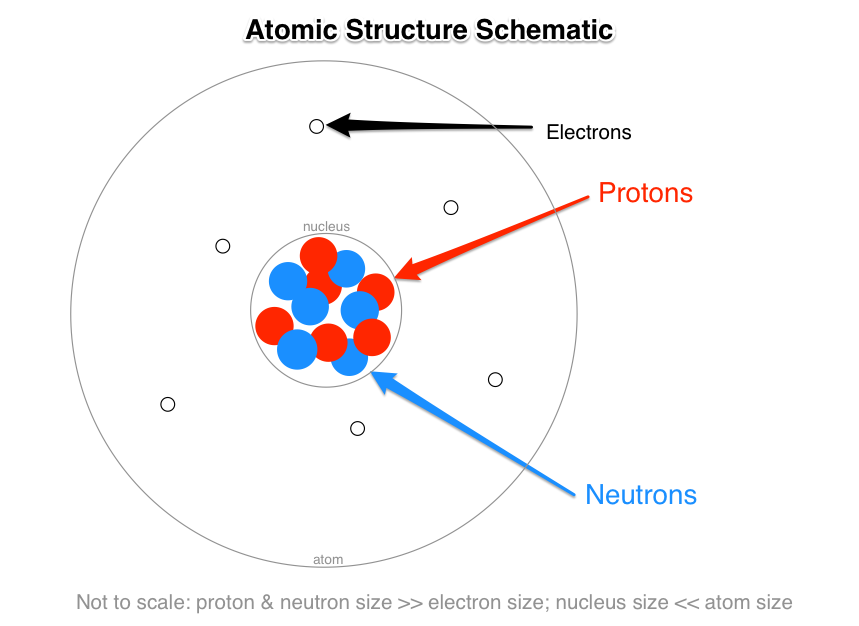
An atom is a smallest particle of matter. Atoms are made up of sub-atomic particles called neutrons electrons and protons. Protons and neutrons are grouped together in the nucleus of an atom while electrons orbit about the nucleus.
The protons and neutrons are packed together into the center of the atom which is called the nucleus and the electrons which are very much smaller whizz around the outside. Describe the electrical charge mass and location of the three subatomic particles in an atom. All the atoms that give rise to an element are similar and are chemically.
Atoms are composed of three main subatomic particles. Protons have a positive charge. Nucleus orbital energy level isotope and ion.

What Is Meant By Subatomic Particle Give Brief Information Of Three Subatomic Particles With Brainly In

The Atom Is Made Of 3 Subatomic Particles The Subatomic Particle Found In The Nucleus With A Positive Charge Is
What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic
No comments for "Describe the Electrical Charges of the Three Subatomic Particles"
Post a Comment